Delve into the fascinating world of ions with our comprehensive guide to formation of ions worksheet answers. Discover the secrets of ion formation, explore their diverse applications, and gain a deeper understanding of the fundamental principles that govern their behavior.
Unravel the mysteries of ion formation, from the loss and gain of electrons to the influence of electronegativity. Learn how ions shape our world, from maintaining electrolyte balance in our bodies to powering batteries and purifying water. Dive into the captivating realm of ions and unlock their potential.
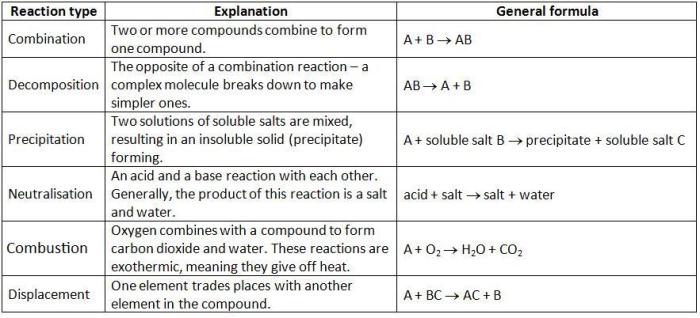
Types of Ions
Atoms can lose or gain electrons to form ions. An ion is an atom or molecule that has lost or gained one or more electrons, resulting in a net electrical charge.
There are two main types of ions:
- Cations: Positively charged ions that form when an atom loses one or more electrons.
- Anions: Negatively charged ions that form when an atom gains one or more electrons.
The charge of an ion is determined by the number of electrons lost or gained. For example, an atom that loses one electron has a +1 charge, while an atom that gains one electron has a -1 charge.
Formation of Cations
Cations are positively charged ions formed when an atom loses one or more electrons. This process occurs when the number of protons in an atom exceeds the number of electrons, resulting in an overall positive charge.
The formation of cations involves the following steps:
- Electron Loss:An atom loses one or more electrons, typically from its outermost energy level.
- Positive Charge:The loss of electrons creates an imbalance between protons and electrons, resulting in a net positive charge.
- Cation Formation:The positively charged atom becomes a cation.
The charge of a cation is determined by the number of electrons lost. For example, an atom that loses one electron forms a cation with a +1 charge, while an atom that loses two electrons forms a cation with a +2 charge.
Examples of Common Cations
- Sodium (Na) loses one electron to form Na +.
- Potassium (K) loses one electron to form K +.
- Calcium (Ca) loses two electrons to form Ca 2+.
- Aluminum (Al) loses three electrons to form Al 3+.
- Iron (Fe) can lose two or three electrons to form Fe 2+or Fe 3+, respectively.
Formation of Anions

Anions are negatively charged ions formed when an atom gains one or more electrons. The process of anion formation is known as electron gain.
Impact of Electron Gain on Atomic Structure
When an atom gains electrons, its electronic configuration changes. The number of electrons in the outermost shell increases, making the atom more stable. The increased electron count also results in a decrease in the atomic radius and an increase in the ionic radius.
Common Anions and Neutral Atoms
- Chloride ion (Cl-) from chlorine atom (Cl)
- Oxide ion (O2-) from oxygen atom (O)
- Sulfide ion (S2-) from sulfur atom (S)
- Nitride ion (N3-) from nitrogen atom (N)
- Fluoride ion (F-) from fluorine atom (F)
Electronegativity and Ion Formation
Electronegativity plays a crucial role in determining the formation of ions. Electronegativity refers to the ability of an atom to attract electrons towards itself. The higher the electronegativity of an atom, the stronger its ability to attract electrons.
In the formation of ions, when two atoms with different electronegativities come together, the atom with higher electronegativity attracts electrons from the atom with lower electronegativity. This results in the formation of ions, with the atom that loses electrons becoming a positively charged cation, and the atom that gains electrons becoming a negatively charged anion.
Electronegativity Differences and Ion Formation
The difference in electronegativity between atoms determines the type of ion formed. A large difference in electronegativity leads to the formation of ionic bonds, where one atom completely transfers electrons to the other. A smaller difference in electronegativity leads to the formation of polar covalent bonds, where electrons are shared between the atoms, but one atom has a greater share of the electrons.
Examples of Electronegativity and Ion Formation
For example, in the formation of sodium chloride (NaCl), sodium (Na) has a low electronegativity of 0.9, while chlorine (Cl) has a high electronegativity of 3.0. The large difference in electronegativity causes sodium to completely transfer its valence electron to chlorine, resulting in the formation of Na+ and Cl- ions.
In contrast, in the formation of water (H2O), hydrogen (H) has an electronegativity of 2.1, while oxygen (O) has an electronegativity of 3.4. The smaller difference in electronegativity results in the formation of a polar covalent bond, where oxygen has a greater share of the electrons.
Applications of Ion Formation: Formation Of Ions Worksheet Answers
Ion formation plays a crucial role in numerous aspects of everyday life, from maintaining the balance of electrolytes in the body to facilitating various chemical reactions.
In the field of chemistry, ions are essential for understanding and manipulating chemical reactions. For instance, the formation of ionic compounds, such as sodium chloride (NaCl), involves the transfer of electrons between atoms, resulting in the formation of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-).
These ions can then interact with each other through electrostatic forces to form stable compounds.
Biology
In biology, ions are involved in a wide range of processes, including the regulation of nerve impulses, muscle contraction, and the maintenance of fluid balance. For example, the movement of sodium and potassium ions across cell membranes is essential for generating electrical signals in nerve cells.
Similarly, the contraction of muscles is triggered by the release of calcium ions from the sarcoplasmic reticulum, which initiates a cascade of events leading to muscle fiber shortening.
Medicine
In medicine, ions are utilized in various applications, such as electrolyte replacement therapy, pH regulation, and drug delivery. Electrolyte solutions, containing ions such as sodium, potassium, and chloride, are used to replenish lost electrolytes in patients suffering from dehydration or electrolyte imbalances.
pH regulation is also important in medicine, as many physiological processes are sensitive to pH changes. Ions can be used to adjust the pH of solutions, such as in the treatment of acidosis or alkalosis.
Electrolyte Balance, Formation of ions worksheet answers
Maintaining the balance of electrolytes in the body is crucial for overall health. Electrolytes, such as sodium, potassium, calcium, and magnesium, are essential for various bodily functions, including nerve transmission, muscle contraction, and fluid regulation. Imbalances in electrolyte levels can lead to a range of health issues, such as muscle cramps, fatigue, and even life-threatening conditions like cardiac arrhythmias.
Questions and Answers
What is an ion?
An ion is an atom or molecule that has lost or gained electrons, resulting in a net electrical charge.
How are cations formed?
Cations are formed when an atom loses one or more electrons, resulting in a positive charge.
How are anions formed?
Anions are formed when an atom gains one or more electrons, resulting in a negative charge.
What is electronegativity?
Electronegativity is a measure of an atom’s ability to attract electrons.
What are some applications of ion formation?
Ion formation has numerous applications, including batteries, water purification, and the maintenance of electrolyte balance in living organisms.