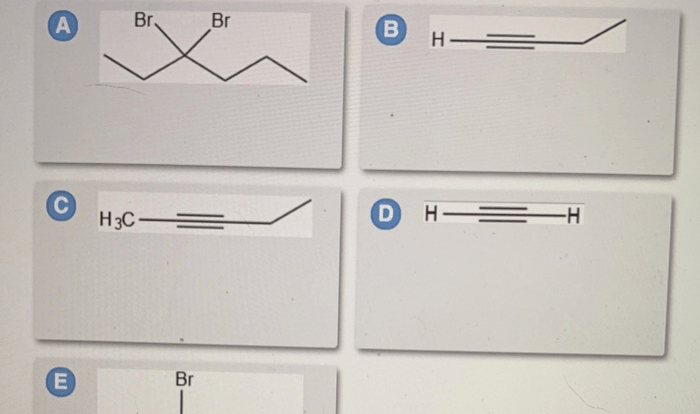
Provide an acceptable name for the alkane shown below. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming alkanes, which are saturated hydrocarbons with only single bonds between carbon atoms. These rules ensure consistency and clarity in chemical nomenclature, enabling scientists and researchers to communicate effectively about organic compounds.
This guide will delve into the IUPAC nomenclature rules for alkanes, providing a comprehensive understanding of how to assign systematic names to these compounds. We will explore the structure of alkanes, their physical and chemical properties, and their diverse applications in various industries.
IUPAC Nomenclature Rules
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming alkanes. These rules are based on the structure of the alkane and the number of carbon atoms in the molecule.
Applying the Rules
To name an alkane using IUPAC rules, follow these steps:
- Identify the parent chain. The parent chain is the longest continuous chain of carbon atoms in the molecule.
- Number the carbon atoms in the parent chain. Start at one end of the chain and number the carbon atoms in order to the other end.
- Identify the alkyl groups attached to the parent chain. Alkyl groups are smaller hydrocarbon groups that are attached to the parent chain.
- Name the alkyl groups. Alkyl groups are named using the suffix “-yl”. The name of the alkyl group is based on the number of carbon atoms in the group.
- Combine the names of the alkyl groups and the parent chain to form the name of the alkane.
Exceptions to the Rules
There are a few exceptions to the IUPAC rules for naming alkanes. These exceptions include:
- Branched alkanes. Branched alkanes are alkanes that have alkyl groups attached to the parent chain. The IUPAC rules for naming branched alkanes are slightly different from the rules for naming unbranched alkanes.
- Cyclic alkanes. Cyclic alkanes are alkanes that form a ring. The IUPAC rules for naming cyclic alkanes are different from the rules for naming unbranched and branched alkanes.
Alkane Structure
Alkanes are hydrocarbons that have only single bonds between the carbon atoms. The carbon atoms in alkanes are arranged in a tetrahedral shape, which gives alkanes their characteristic three-dimensional structure.
Shape of Alkanes
The shape of an alkane depends on the number of carbon atoms in the molecule. Small alkanes, such as methane and ethane, are relatively symmetrical. As the number of carbon atoms increases, the shape of the alkane becomes more elongated.
The following table shows the shapes of different alkanes:
| Number of Carbon Atoms | Shape |
|---|---|
| 1 | Tetrahedral |
| 2 | Tetrahedral |
| 3 | Staggered |
| 4 | Sawhorse |
| 5 | Zigzag |
| 6 | Chair |
Alkane Properties
Alkanes are nonpolar, saturated hydrocarbons. They are generally unreactive and have low melting and boiling points. The physical and chemical properties of alkanes change with increasing molecular weight.
Physical Properties
The physical properties of alkanes include:
- Melting point: The melting point of alkanes increases with increasing molecular weight.
- Boiling point: The boiling point of alkanes increases with increasing molecular weight.
- Density: The density of alkanes increases with increasing molecular weight.
- Solubility: Alkanes are insoluble in water.
Chemical Properties
The chemical properties of alkanes include:
- Combustion: Alkanes are highly flammable and burn in air to produce carbon dioxide and water.
- Halogenation: Alkanes can react with halogens to form alkyl halides.
- Nitration: Alkanes can react with nitric acid to form nitroalkanes.
- Sulfonation: Alkanes can react with sulfuric acid to form sulfonic acids.
Alkane Reactions
Alkanes can undergo a variety of reactions, including combustion, halogenation, nitration, and sulfonation. The mechanisms of these reactions vary depending on the specific reaction.
Combustion
Combustion is the most common reaction of alkanes. When an alkane burns, it reacts with oxygen to produce carbon dioxide and water.
Halogenation
Halogenation is a reaction in which an alkane reacts with a halogen to form an alkyl halide. The mechanism of halogenation is a radical chain reaction.
Nitration, Provide an acceptable name for the alkane shown below.
Nitration is a reaction in which an alkane reacts with nitric acid to form a nitroalkane. The mechanism of nitration is an electrophilic aromatic substitution reaction.
Sulfonation
Sulfonation is a reaction in which an alkane reacts with sulfuric acid to form a sulfonic acid. The mechanism of sulfonation is an electrophilic aromatic substitution reaction.
Applications of Alkanes: Provide An Acceptable Name For The Alkane Shown Below.
Alkanes are used in a wide variety of applications, including:
Fuels
Alkanes are the primary components of natural gas and petroleum. Natural gas is used to heat homes and businesses, while petroleum is used to produce gasoline, diesel fuel, and other fuels.
Solvents
Alkanes are used as solvents in a variety of industries, including the chemical, pharmaceutical, and food industries.
Lubricants
Alkanes are used as lubricants in a variety of applications, including automotive, industrial, and marine applications.
FAQ Resource
What is the IUPAC nomenclature system?
The IUPAC nomenclature system is a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC) for naming inorganic and organic compounds. It provides a systematic and standardized approach to naming chemical compounds, ensuring consistency and clarity in scientific communication.
How do I name an alkane using IUPAC nomenclature?
To name an alkane using IUPAC nomenclature, follow these steps:
- Identify the parent chain, which is the longest continuous chain of carbon atoms in the molecule.
- Determine the root name of the alkane based on the number of carbon atoms in the parent chain.
- Identify and name any alkyl groups (branched chains) attached to the parent chain.
- Assign numbers to the carbon atoms in the parent chain, starting from the end closest to the first alkyl group.
- Combine the root name, alkyl group names, and numbers to form the complete IUPAC name of the alkane.
What are the exceptions to the IUPAC nomenclature rules for alkanes?
There are a few exceptions to the IUPAC nomenclature rules for alkanes, including:
- Common names are sometimes used for simple alkanes (e.g., methane, ethane, propane, butane).
- Cyclic alkanes (cycloalkanes) have their own set of naming rules.
- Alkanes with multiple alkyl groups may have more than one acceptable IUPAC name.