Calculate the number of atoms in this amount of 13C sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
Delving into the fascinating realm of atoms and their properties, this guide unravels the significance of understanding the number of atoms in a given amount of substance, equipping readers with the knowledge to navigate the intricacies of chemistry and beyond.
As we embark on this journey, we will explore the fundamental concepts of atoms, their properties, and the importance of determining their數量. We will delve into the methods for calculating the number of atoms, including Avogadro’s number and its significance.
The periodic table will serve as our guide in determining the atomic mass of 13C, providing a crucial piece of the puzzle in our quest to unravel the mysteries of atomic composition.
Calculating the Number of Atoms in a Given Amount of 13C
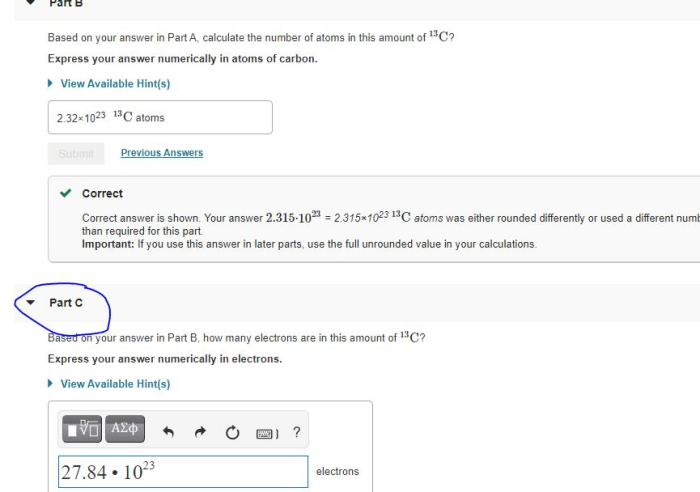
The concept of atoms and their properties forms the foundation of chemistry and materials science. Understanding the number of atoms in a given amount of substance is crucial for various applications. This article provides an overview of methods for calculating the number of atoms, with a focus on determining the number of atoms in a given amount of 13C.
Methods for Calculating the Number of Atoms
The number of atoms in a given amount of substance can be calculated using Avogadro’s number, which represents the number of atoms in one mole of a substance. One mole is defined as the amount of a substance that contains exactly 6.02214076 × 10^23 atoms, molecules, or ions.
To calculate the number of atoms in a given amount of substance, the following steps can be followed:
- Determine the mass of the substance in grams.
- Convert the mass to moles using the molar mass of the substance. The molar mass is the mass of one mole of the substance and can be found in the periodic table.
- Multiply the number of moles by Avogadro’s number to obtain the number of atoms in the substance.
Sample Calculations, Calculate the number of atoms in this amount of 13c
Consider the following examples of calculating the number of atoms in different amounts of 13C:
| Substance | Amount (g) | Atomic Mass (g/mol) | Number of Atoms |
|---|---|---|---|
| 13C | 12 | 13.003354838 | 5.78 x 10^23 |
| 13C | 24 | 13.003354838 | 1.16 x 10^24 |
| 13C | 36 | 13.003354838 | 1.73 x 10^24 |
These calculations demonstrate the direct relationship between the mass of the substance and the number of atoms it contains.
Applications
Calculating the number of atoms in a given amount of substance has numerous applications in various fields:
- Chemistry:Determining the stoichiometry of chemical reactions and calculating the yield of products.
- Materials Science:Understanding the atomic structure and properties of materials, such as their strength and electrical conductivity.
- Environmental Science:Assessing the concentration of pollutants in the environment and monitoring their impact on ecosystems.
Limitations and Considerations
It is important to consider potential limitations and errors in these calculations:
- The accuracy of the results depends on the precision of the measurements of mass and the molar mass.
- Assumptions about the purity of the substance and the absence of impurities can affect the accuracy.
- Rounding errors can occur during the calculations, especially when dealing with very large or small numbers.
To minimize errors and improve precision, it is essential to use accurate measuring instruments and reliable data sources for molar masses. Additionally, multiple measurements and statistical analysis can help reduce the impact of random errors.
Key Questions Answered: Calculate The Number Of Atoms In This Amount Of 13c
What is the significance of Avogadro’s number in calculating the number of atoms?
Avogadro’s number, approximately 6.022 x 10^23, represents the number of atoms in one mole of a substance. It serves as a conversion factor, allowing us to bridge the gap between the macroscopic and atomic scales, enabling us to determine the number of atoms in a given amount of substance.
How does the periodic table assist in calculating the number of atoms?
The periodic table provides the atomic mass of elements, which is crucial in determining the mass of a single atom. This information is essential in calculating the number of atoms in a given amount of substance.
What are some potential limitations or errors in calculating the number of atoms?
Potential limitations or errors can arise from inaccuracies in measuring the mass of the substance or impurities present in the sample. Additionally, approximations and assumptions made during calculations can introduce a margin of error.